含镍量为12% ~ 15%的碱性锌镍合金电镀工艺
含镍量为12% ~ 15%的碱性锌镍合金电镀工艺
发布时间:2018.02.06 新闻来源:江苏梦得新材料科技有限公司 浏览次数:
汤新生[1],杭冬良,周佩佩
(江苏梦得电镀化学品有限公司,江苏 丹阳 212341)
摘要:本文采用新的添加剂和络合剂实现了含镍量12%~15%(质量分数)的碱性锌镍合金电镀,并通过重点研究镀液各成分对镀层性能的影响规律,优选出了新的碱性锌镍合金电镀工艺和镀液配方。采用不同的钝化液配方及相应的工艺对镀层进行钝化处理。通过中性盐雾试验对钝化层的耐腐蚀性检测,筛选出合适的钝化工艺进一步提高了镀层的耐腐蚀性。
关键词:碱性锌镍合金,添加剂,钝化,耐蚀性
Process for electroplating Zinc-Nickel Alloy with 12%-15% nickel from an alkaline bath
Tang xin sheng1, Hang dong liang , Zhou pei pei
(Jiangsu Mengde Electroplate Chemical Products CO.,Ltd. danyang212341,Jiangsu,China)
Abstract: In this paper, electroplating Zinc-Nickel alloys which the content of nickel was in 12% ~15%(by weight) had realized by selecting new additive and complexant , and the rule of electroplatingsolution effecting the performance of coating had been put on the emphasis. The new process condition and solutions of electroplating Zinc-Nickel alloys had been obtained and researched from all aspects. The passivation of Zinc-Nickel alloys corrosion resistance had been researched by the neutral salt spray test, obtained the proper passivation technology and further improved the corrosion resistance of coating.
Key words: Alkaline Zn-Ni Alloy, Additive, Passivation, Corrosion resistance
1.前言
随着现代工艺和科学技术的迅速发展,人们对防护性镀层的质量要求也越来越高,电镀随之迅猛发展。锌镍合金因其突出的性能,在日本、美国、欧洲已得到了广泛应用。可以肯定,锌镍合金电镀具有广阔的发展前景。
锌镍合金镀液主要分酸性体系和碱性体系。由于碱性镀液的分散能力好,镀层厚度均匀,对设备和工件腐蚀性较小,工艺操作容易,成本较低等优点,近年来已引起国内外研究者的重视。为了进一步提高碱性锌镍合金镀层的耐蚀性和装饰性,一般都需要钝化处理。经过钝化处理的镀层耐蚀性能提高多倍,作为防护性镀层是非常必要的。
锌镍合金镀层具有比锌镀层高得多的抗腐蚀性,其优异的耐蚀性能倍受广大研究者的关注。本实验对纯锌镀层和不同镍含量的锌镍合金镀层进行了中性盐雾实验。对比发现镍含量7%~9%的锌镍合金耐蚀性是锌镀层的3倍以上;含镍量13%左右的锌镍合金镀层是锌镀层的5倍以上,其具有较好的耐蚀性能。锌镍合金的耐蚀性与含镍量的关系见图1-1所示。
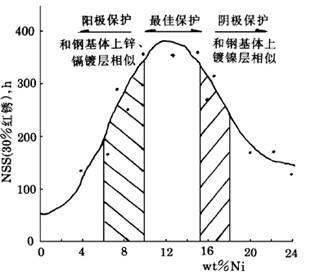
图1-1镀层中镍含量与耐蚀性的关系(未钝化)
本文介绍新近研制的MNX-2011添加剂和MNX-2011络合剂在碱性锌镍合金电镀工艺中的应用,并在此基础上对镀层进行钝化处理。实践证明该工艺稳定,溶液维护简单,便于工业化生产。
2.实验方法
2.1基材与设备
基材采用冷轧铜试片(65×100×0.1mm³)或钢铁紧固件;阳极由锌板和不锈钢板(面积比为1:4)组成。
主要设备有:DDZ-10A型调压变压整流器(浙江省绍兴市合力整流器厂),YWX/F-150型盐雾腐蚀试验箱(江苏安特稳科技有限公司),BS-1500L型电子天平(上海友声衡器有限公司),霍尔槽,恒温水浴装置等。
2.2电镀工艺过程
2.2.1碱性电镀锌镍合金镀液组成及工艺条件
氧化锌/(g/L) 7-10
六水合硫酸镍/(g/L) 5-10
氢氧化钠/(g/L) 120-140
MNX-2011络合剂/(ml/L) 100
MNX-2011主光剂/(ml/L) 2-4
MNX-2011柔软剂/(ml/L) 4-6
MNX-2011辅助剂/(ml/L) 10-20
温度/℃ 15-30
阴极电流密度/A/dm2 0.5-4
阳极 锌板与不锈钢板并用(面积比为1:4)
搅拌 阴极移动
过滤 连续,每小时2-3次循环
2.2.2工艺流程
化学除油—流水清洗—酸洗—流水清洗—弱腐蚀活化—流水清洗—电镀。
2.2.3电镀实验
以电镀小槽试验考察主盐,添加剂,络合剂等成分在一个比较宽的电流密度范围内,对镀层外观的影响状况。本试验采用2L的电镀小槽,工作电流为0.5~2A,时间为5~20min,温度控制在15-25℃。
2.3镀液的性能测试
2.3.1镀液稳定性测试
在实际生产中,镀液是否能够长期稳定(要求不出现沉淀)关系着生产能否连续进行以及产品质量能否一致合格。因此,需要对镀液的稳定性进行评定 [1]。
试验一:将镀液加热到60~70℃,络合剂没有出现分解现象,冷却后,补充水分后,电镀仍能正常进行。
试验二:将镀液静置一个星期左右,镀液有没有出现浑浊,补充水分后,电镀也能正常进行。
2.3.2镀液成分分析
镀液中镍离子含量的分析采用丁二酮肟法[2];分光光度值在525nm[3]下测分光度,再计算分光值与镀液中镍含量的关系。锌离子和镍离子总量的分析以紫脲酸铵为指示剂,用EDTA 滴定法,锌离子的含量就是总量减去镍离子含量即得。
2.4镀层性能测试
2.4.1镀层镍含量的分析
1、分光光度计法
镀层中镍含量的分析方法与镀液的分析方法类似。镀层先沉积在铜片上再用20%(体积分数)的硫酸褪镀,分析褪镀液中金属离子含量。相关试剂的配制参考文献[4]。
2、能谱仪分析
采用美国FEI 公司生产的QUANTA200 型扫描电子显微镜能谱分析仪,分析验证合金镀层的镍含量。
3、x 射线荧光分析
采用英国牛津公司生产的MDX1000 型X 射线荧光光谱仪,分析验证镀层中的各种金属含量。
2.4.2镀层厚度测试
1、称重法
本试验中采用溶解称重法[5]对锌-镍合金镀层测试厚度,具体操作如下:
(1) 以铜片为阴极,电镀锌-镍合金10 分钟,并测量试片的镀层面积S;
(2) 称量试片(带有镀层)重量W1;
(3) 以1:5 的硫酸将试片的镀层充分溶解掉,再次称量铜片(无镀层)重量W2,然后使用721-B 型分光光度计测出吸光度,并计算镀层的镍含量Ni%;
(4) 以公式ρZn-Ni=ρNi×Ni%+ρZn×Zn%计算锌-镍合金镀层的密度ρZn-Ni;
(5) 按公式h=(W1-W2)·S/ρZn-Ni 计算试片镀层的厚度。
2、x 射线荧光分析
采用英国牛津公司生产的MDX1000 型X 射线荧光光谱仪,测镀层的厚度。
2.4.3镀层盐雾实验测试
试验采用江苏安特稳科技有限公司的YWX/F-150型盐雾试验箱,按照国家标准GB/T10125-1997《人造气氛腐蚀试验 盐雾试验》进行中性盐雾试验(NSS)。
将NaCl溶于电导率不超过2µs/cm的蒸馏水或去离子水中,其浓度为50g/L±5g/L。在25℃时,配制的溶液密度在1.0255-1.0400范围内,调整pH在6.5-7.2之间,试验箱的温度为35℃;经24h喷雾后,盐雾的沉降速度为每80cm2面积上为1~2ml/h;连续喷雾。
3.各成分的作用及其对镀层性能的影响
3.1主盐的作用及其影响
氧化锌是镀液中金属锌离子的来源,锌离子浓度的多少直接影响镀层中镍含量。镀液中Zn2+、Ni2+质量比以1~3.5为佳,使镀层中含镍的质量分数在14%~11%之间。镀液中锌离子浓度高,镀层显白色,低电流区镀层显灰白色,镀层中镍含量降低,耐腐蚀性差。
硫酸镍是镀液中镍离子的来源,与锌离子共同沉积,形成一定镍含量的耐腐蚀锌镍合金镀层。适当提高镍离子浓度有利于提高镀层中镍含量,但镀液中镍离子浓度过高,同时络合剂配比过少,则会使镀层低电流密度区发黑。因此在电镀过程中,如镀层低区发黑,应考虑镀液中是否镍离子浓度过高,需补加适当的络合剂来调整。

图3-1镀液中Zn2+/Ni2+质量比对镀层中含镍量的影响
镀液中Zn2+/ Ni2+质量比镀层外观影响不大,但对镀层中含镍量影响较大,参看图3-1。针对镀层中镍含量控制13%左右,应控制Ni2+/ Ni2++Zn2+为0.16~0.32为优。
3.2络合剂的作用及其影响
常用的络合剂有柠檬酸盐,酒石酸盐,葡萄糖酸盐和多元醇以及有机胺等。通过实验对比发现,MNX-2011络合剂(由有机胺类和羧酸盐类物质组成)对镀液的稳定性和镀层的性能都有较好效果。在电镀过程中,络合剂过少则镍离子过多,会使镀层发黑,特别是使低电流密度区发黑,此时应适当补加络合剂来改善。
表3-1各种络合剂镀液的稳定性与镀层的含镍量
|
配方
|
ρ(NiSO4·6H2O)/
(g/L)
|
配位剂
|
镀液稳定性
|
镀层含镍量/%
|
|
1
|
28
|
MNX-2011 100 mL/L
|
稳定
|
24
|
|
2
|
5
|
乙二胺30 g/L + 三乙醇胺40 mL/L
|
不稳定
|
12.5
|
|
3
|
3
|
二乙烯三胺12 g/L + 三乙醇胺3.5 mL/L
|
不稳定
|
7.5
|
|
4
|
5
|
MNX-2011 100 mL/L
|
稳定
|
13.4
|
注:镀液中ZnO 10 g/L,NaOH 135 g/L。
由表3-1可知,镀液中NiSO4.6H2O浓度跟镀层含镍量正相关,镀液中NiSO4.6H2O浓度较高时(配方1中NiSO4.6H2O为28g/L)镀层的镍含量为24%;当溶液中NiSO4.6H2O浓度较低时(配方3中NiSO4.6H2O为3.5g/L)得到镀层镍含量为7.5%。易挥发的有机物作为镍的络合剂,会使镀液不稳定,配方2中的乙二胺具有挥发性,以其为络合剂的镀液受热后变色严重,出现絮状悬浮物,而且获得的镀层外观发黑。络合剂的络合能力对镀层含镍量有影响。一般而言,与镍离子形成不稳定系数小的物质,其络合能力较好。配方1的镀液稳定性较好,且镀层光亮,对应的镀层含镍量可通过镀液中的锌、镍离子比调整。故确定络合剂MNX-2011作为进一步研究的镀液络合剂体系。配方4的镀液稳定性较好,镀层含镍量通过检测达到13.4%,此工艺基本合乎要求。
络合剂对合金成分的影响见图3-2络合剂的主要作用是防止金属盐水解和稳定镀液;促进阳极正常溶解;增加阴极极化和改善镀层结晶。
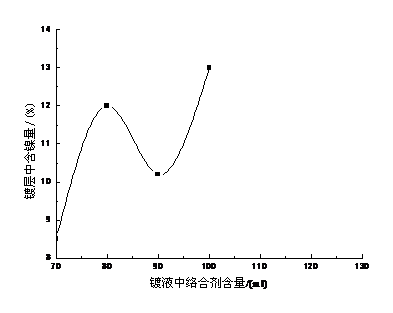
图3-2合剂对合金成分的影响
3.3添加剂的作用及其影响
对镀层具有细化结晶,整平和光亮作用。通常使用的有芳香醛,有机胺以及环氧氯丙烷和有机胺的合成物等。本试验采用杂环类有机物和有机胺类合成物作为添加剂。MNX-2011添加剂的影响见图3-3,对镀层中含镍量也有一定影响。
MNX-2011添加剂由主光剂,柔软剂,辅助剂组成。主光剂起整平光亮作用,过多使镀层乌黑,但有脆性,过少则整平亮度下降。柔软剂主要使镀层柔软,消除脆性,同时具有阴极极化作用,增强走位作用,使镀层均匀分散。过多易产生光亮条纹,过少阴极极化不强,走位不行,且消除镀层脆性的能力下降。辅助剂主要起增亮作用及阴极极化作用,增强走位能力,增加辅助剂的用量可使镀层发白。
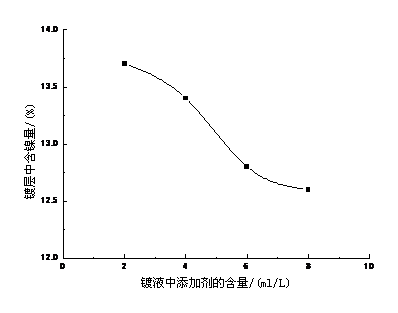
图3-3镀液中添加剂对镀层中含镍量的影响
4.锌镍合金镀层的耐蚀性
4.1中性盐雾试验结果
镀层含镍量对未钝化Zn-Ni合金镀层耐蚀性能的影响如表4-1。
表4-1不同含镍量的镀层中性盐雾试验结果
|
镀层含镍量/(%)
|
试验结果
|
|
0
|
试验3h试件表面出现了黑色腐蚀点;试验6h后试件大面积出现黑色腐蚀点;10h后试件被白色腐蚀产物覆盖。
|
|
7.5
|
试验6h后试件已发生腐蚀,白色腐蚀点很多;试验24h后,试件表面全部被白色腐蚀产物覆盖。
|
|
24
|
试验16h后试件白色腐蚀点增多;试验48h后,试件表面布满白色腐蚀点。
|
|
13.4
|
试验24h后试件已发生腐蚀,出现极少量的白色腐蚀点;96h后,试件表面有较少白色腐蚀点。
|
锌镍合金镀层的腐蚀产物主要是ZnCl2.4Zn(OH)2,该产物均匀致密地覆盖在表面上,且不容易导电,对镀层起保护作用;而锌镀层的腐蚀产物主要是ZnO,其结构疏松,起不到保护作用。同时,由于Zn-Ni合金中有镍的存在,对腐蚀反应过程和腐蚀产物中产生了有利的影响,使腐蚀反应有利于形成Zn(OH)2保护层。
由表4-1可见,含镍量为13.4%的Zn-Ni合金镀层耐蚀性能优于其它含镍量的Zn-Ni合金镀层;含镍量24%的Zn-Ni合金镀层耐蚀性稍优于含镍量7.5%的Zn-Ni合金镀层;而碱性纯锌镀层(含镍量为0)的耐蚀性较差。
下图4-1不同含镍量镀层经中性盐雾腐蚀后镀层表面的腐蚀情况。
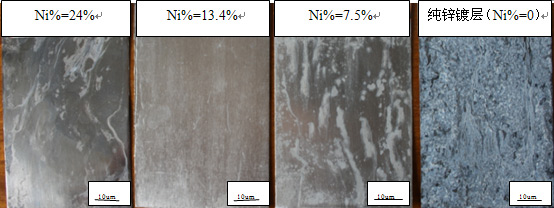
图4-1不同镍含量镀层的腐蚀情况
4.2腐蚀后镀层的微观形貌
图4-2盐雾腐蚀后镀层的微观形貌[6]。由图4-2可看出,纯锌镀层腐蚀表面疏松(见图4-2a),用1%醋酸水溶液清洗镀层表面腐蚀产物。发现锌镍镀层表面产生了大量微裂纹(见图4-2b)作剖面金相观察。裂纹是贯穿镀层的,而锌镀层则未发现。
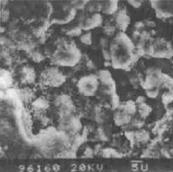
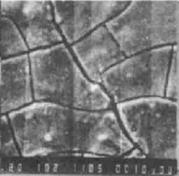
(a) (b)
图4-2盐雾腐蚀试验后镀层微观形貌
5.锌镍合金镀层的钝化工艺
5.1镀层钝化工艺
锌镍合金上形成彩色钝化膜比锌镀层上要困难的多,且随合金镀层中含镍量增加,则越加困难,一般合金中含镍量在10%以内,钝化还比较容易,含镍量在13%左右时,钝化则比较困难,当含镍量超过16%,则很难钝化。针对MNX-2011工艺锌镍合金镀层含镍量13%左右,通过钝化层外观颜色比较和中型盐雾试验耐腐蚀对比。筛选出以下三种钝化工艺,见表5-1几种钝化工艺的成分及工艺条件。
表5-1几种钝化工艺的成分及工艺条件
|
成分及工艺条件
|
彩虹色钝化
|
白色(本色)钝化
|
|
|
铬酐/(g/L)
|
5-10
|
15
|
5
|
|
促进剂A/(g/L)
|
|
|
2
|
|
硫酸/(ml/L)
|
10
|
|
|
|
增白剂/(g/L)
|
|
0.5
|
|
|
促进剂B/(g/L)
|
2
|
|
|
|
pH值
|
1.4
|
1.0
|
1.5
|
|
工作温度℃
|
30
|
20-30
|
20-30
|
|
钝化时间/s
|
10
|
15-35
|
15-35
|
5.2镀层钝化膜的耐蚀性
含镍量为13.4%镀层经钝化处理后的中性盐雾试验结果如下表5-2所示。
表5-2镀层钝化后的中性盐雾试验结果
|
镀层处理
|
试验结果
|
|
彩色钝化
|
试验96h后试件表面出现少量灰白色腐蚀点;试验290h后,试件表面出现白色腐蚀点。
|
|
白钝化
|
试验96h后试件表面出现少量灰白色腐蚀点;试验300h后,试件表面出现白色腐蚀点。
|

图5-1不同钝化腐蚀后的工件
未钝化时,中性盐雾试验24h后试件已出现少量的白色腐蚀点;经钝化处理后,96h后才出现灰白色腐蚀点。可见,钝化后镀层的耐蚀性能明显优于未经钝化处理的镀层。表5-2表明,不同的钝化工艺有不同的钝化效果,进而导致不同的耐蚀性能,白色钝化膜耐蚀性优于彩色钝化膜。
6.结论
本文采用了碱性锌镍合金镀液体系,获得了含镍量为12~15%的锌镍合金镀层。实验表明,所得锌镍合金镀层具有良好的外观和耐蚀性。采用新的钝化液配方及相应的工艺对镀层进行钝化处理进一步提高了锌镍合金镀层的耐腐蚀性能。
参 考 文 献
[1] 肖作安.硫酸盐体系锌镍合金电沉积规律性的研究[D].武汉:华中师范大学,2005.
[2] 侯燕.碱性锌-镍合金镀液及镀层中锌镍的测定[J].电镀与精饰, 2005, 27(1): 43~45.
[3] 马钦科.元素的分光光度法[M].北京: 地质出版社, 1976: 321~322.
[4] 楼书聪,杨玉玲.化学试剂配制手册[M].江苏科学技术出版社,2002,11.
[5] 沈品华,屠振密.镀锌及锌合金[M]. 北京: 机械工业出版社, 2002: 211~236..
[6] 卢锦堂,陈锦红等.锌镍合金镀层盐雾腐蚀行为的研究[J].材料保护,1997,30(5):8~10.
作者简介:汤新生(1963-),男,江苏省丹阳人,工程师,主要从事电镀工艺研究工作。
作者联系方式:(E-mail)hdl@chinamengde.com
下一篇为您介绍: 处理某厂镍缸老槽液实例,如需了解更多我们的信息,请持续关注。
含镍量为12% ~ 15%的碱性锌镍合金电镀工艺由江苏梦得新材料科技有限公司于2018.02.06整理发布。
转载请注明出处: http://www.gzrfp.cn//techview.asp?ID=269。
上一篇新闻:复合染料型酸性镀铜工艺的研究
下一篇新闻:处理某厂镍缸老槽液实例
相关新闻:
| 2018.02.06 | |
| 2025.04.02 | |
| 2018.02.08 |
相关产品:











